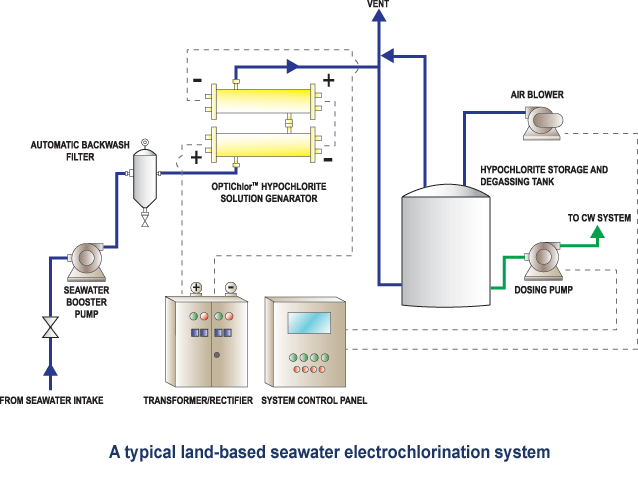
Seawater Electrochlorination
Any industrial application that uses seawater for cooling has the potential for the formation of biofouling from the various organisms lurching in the oceans. Our simple yet innovative systems use the salt from seawater to generate a dilute sodium hypochlorite (bleach) solution which when injected into the seawater cooling circuits, kills bio-growth without the need for handling chlorine gas. The only inputs are seawater and electricity.
The production of hypochlorite (active chlorine) is based on passing a direct current through an aqueous solution of sodium chloride (NaCI), which is totally dissociated to Na+ and Cl-;
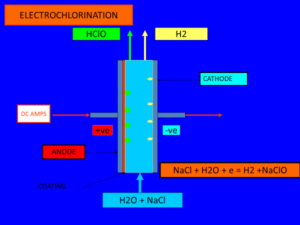 The following chemical and electrochemical reactions occur:
The following chemical and electrochemical reactions occur:
a) Free chlorine is generated at the anode:
2 Cl– → Cl2 + 2 e–
b) Hydrogen is evolved at the cathode with the corresponding formation of hydroxyl ion:
2 H2O + 2 e– → H2 + 2 OH–
c) The overall electrochemical reaction is:
2 Cl– + 2 H2O → Cl2 + H2 + 2 OH–
d) Then chlorine and hydroxyl ions react chemically producing hypochlorite and chloride:
2 OH– + Cl2 → ClO– + Cl– + H2O
e) The overall chemical reaction can be expressed as follows:
2 NaOH + Cl2 → NaClO + NaCl + H2O
In the chemical literature, hypochlorite concentrations are commonly referred to in terms of “available” or “active” chlorine, i.e. the quantity of chlorine having the same oxidizing effect as the hypochlorite when analyzed by standard methods.